Introduction to HFOs
Context
One of the main challenges in epilepsy surgery is accurately identifying the epileptogenic zone responsible for seizures. To achieve this, pre-surgical evaluation often includes intracranial EEG recordings conducted over a hospital stay lasting one to three weeks. Analyzing the resulting large volumes of EEG data is time-consuming and heavily reliant on the expertise of epileptologists.
In standard clinical practice, clinicians typically wait for the patient to experience spontaneous seizures and focus their analysis on the EEG signals immediately preceding the seizure to determine the epileptogenic zone. As a result, a large portion of the interictal (between seizures) EEG data remains unexplored. Furthermore, some patients do not have seizures during their monitoring period. Most critically, only about 60–65% of patients ultimately proceed to surgery, often because the epileptogenic zone could not be clearly localized.
For these reasons, there is growing interest in analyzing EEG features recorded between seizures—known as interictal biomarkers of epilepsy—such as epileptic spikes and, in particular, high-frequency oscillations (HFOs).
High-frequency oscillations (HFOs)
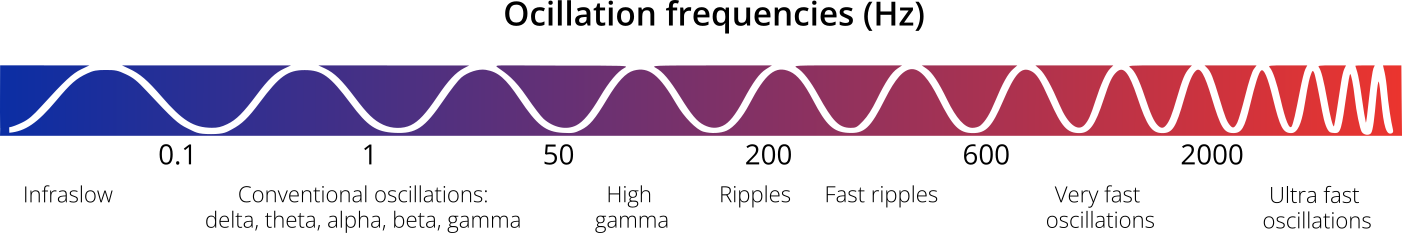
HFOs are brief high-frequency oscillations observed in EEG recordings. They are typically classified into two main categories: ripples (80–200 Hz) and fast ripples (200–600 Hz). In some cases, high-gamma activity (60–120 Hz) and very fast oscillations (600–2000 Hz or higher) are also included under the broader term HFOs (Figure 1). The frequency boundaries between these categories are arbitrary and can vary across studies.
Since the early 2000s (Bragin et al, 1999), HFOs have garnered significant attention as promising biomarkers for identifying the epileptogenic zone (reviewed in Zijlmans et al., 2012). However, their adoption in clinical practice has been limited for several reasons. These include the need for reliable automated detectors to support clinicians, and ongoing debate about the added value of HFOs compared to other established biomarkers. There has recently been a growing interest in combined biomarkers, such as HFOs superimposed on epileptic spikes, which may offer enhanced diagnostic potential.
Figure 1. Range of EEG oscillation frequencies.
Fast ripples
Fast ripples, particularly those in the higher frequency range above 400 Hz, are widely considered to be pathological and have emerged as promising biomarkers for localizing the epileptogenic zone. Unlike ripples, fast ripples are thought to originate predominantly from abnormally hyperexcitable neuronal networks. They are typically defined as short bursts of high-frequency activity composed of at least four consecutive oscillations that clearly stand out from the surrounding EEG background. These events generally last less than 15 milliseconds and have relatively low amplitudes, making them challenging to detect visually. Their spatial specificity, often confined to seizure-generating tissue, makes them particularly valuable for surgical planning. However, detecting fast ripples remains methodologically complex, as filtered transients (such as sharp epileptic spikes) can produce “false” fast ripples (Bénar et al, 2010). Therefore, accurate detection often requires a combination of automated algorithms and expert validation.
Figure 2. Example of a fast ripple (highlighted in red) occurring just before an epileptic spike, as detected by Halyzia. Note the time scale of the window—only 400 ms—to allow clear visualization of the fast ripple. Top panel: raw EEG signal; middle panel: band-pass filtered signal (200–600 Hz); bottom panel: scalogram illustrating the frequency content of the fast ripple. Various features are computed for each detected event, including frequency, amplitude, duration, and entropy. These metrics can be reviewed and exported for further analysis.
Ripples
Ripples (Figure 3) can be classified into two distinct types: physiological ripples, which are associated with normal brain functions such as memory consolidation and sensory processing, and pathological ripples, which are linked to epileptogenic activity. Differentiating between these two forms remains a major challenge in both research and clinical contexts. While they often overlap in frequency range and morphology, some studies (such as Frauscher et al. (2024)) have proposed distinguishing features, including differences in spatial distribution, co-occurrence with epileptic spikes, and behavioral state dependency (e.g., occurring during sleep vs. wakefulness). However, these criteria are not yet universally accepted or sufficiently reliable for routine clinical application.
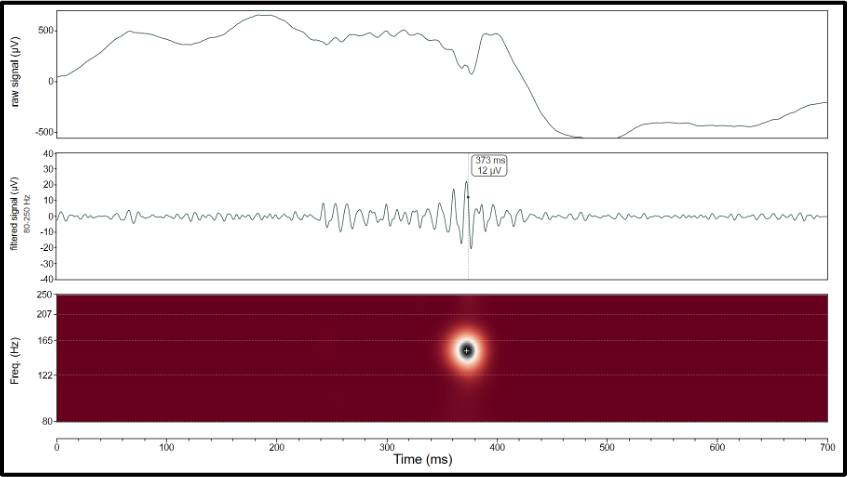
Figure 3. Example of a ripple detected with Halyzia. Beware of the time scale of the window, here set to 700 ms to clearly see the ripple. Top panel: raw signal, middle panel: filtered signal (80-250 Hz), bottom panel: scalogram showing the frequency range of the ripple. Different metrics are calculated for each ripple (frequency, amplitude, duration, entropy, etc.). These can be reviewed and exported.
What can Halyzia® do regarding HFO?
Halyzia includes detectors for ripples and fast ripples. After detection, each event can be reviewed individually to verify its nature and assess its clinical relevance. The results can then be displayed on a topographic map (Figure 4) and exported if needed.
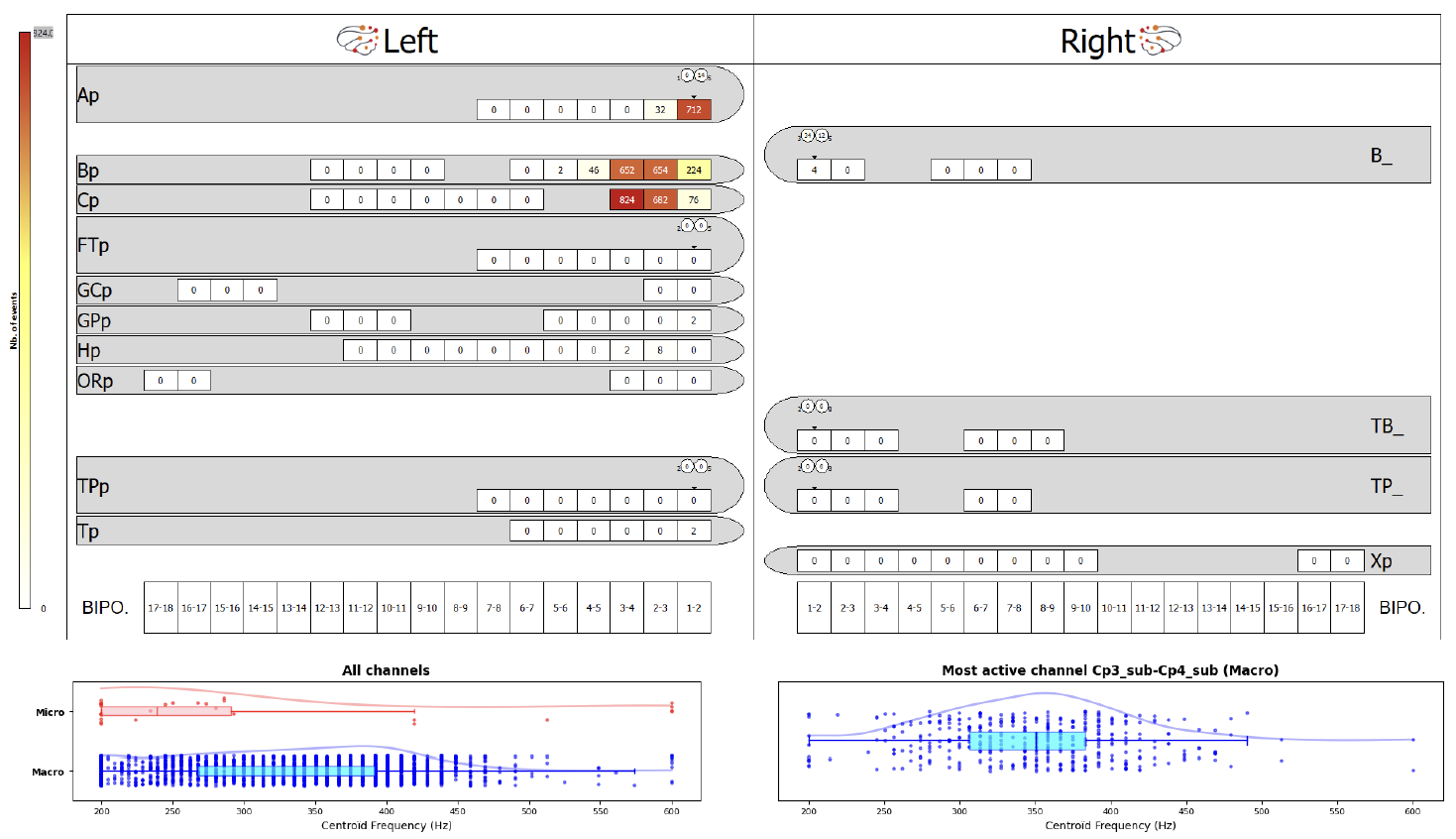
Figure 4. Example of a topographic map. This maps represents implanted electrodes (10 on the left, 4 on the right hemisphere) and the contacts on each electrode. The results indicate that fast ripples were mostly recorded on contacts 1-2-3-4 of electrodes Ap, Bp and Cp. The bottom graphs show the distribution of fast ripple centroids from 200 to 600 Hz.
Authors: Emmanuel Barbeau, Clarissa Baratin - first published May 26th, 2025
A question about this article? Contact us at support_halyzia@avriomedtech.com
References
Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on "false" ripples. Clin Neurophysiol. 2010;121(3):301-310. doi:10.1016/j.clinph.2009.10.019
Bragin A, Engel J Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40(2):127-137. doi:10.1111/j.1528-1157.1999.tb02065.x
Frauscher B, Mansilla D, Abdallah C, et al. Learn how to interpret and use intracranial EEG findings. Epileptic Disord. 2024;26(1):1-59. doi:10.1002/epd2.20190
Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71(2):169-178. doi:10.1002/ana.22548